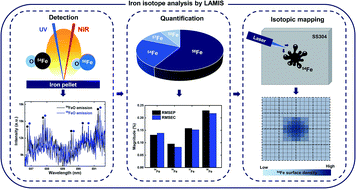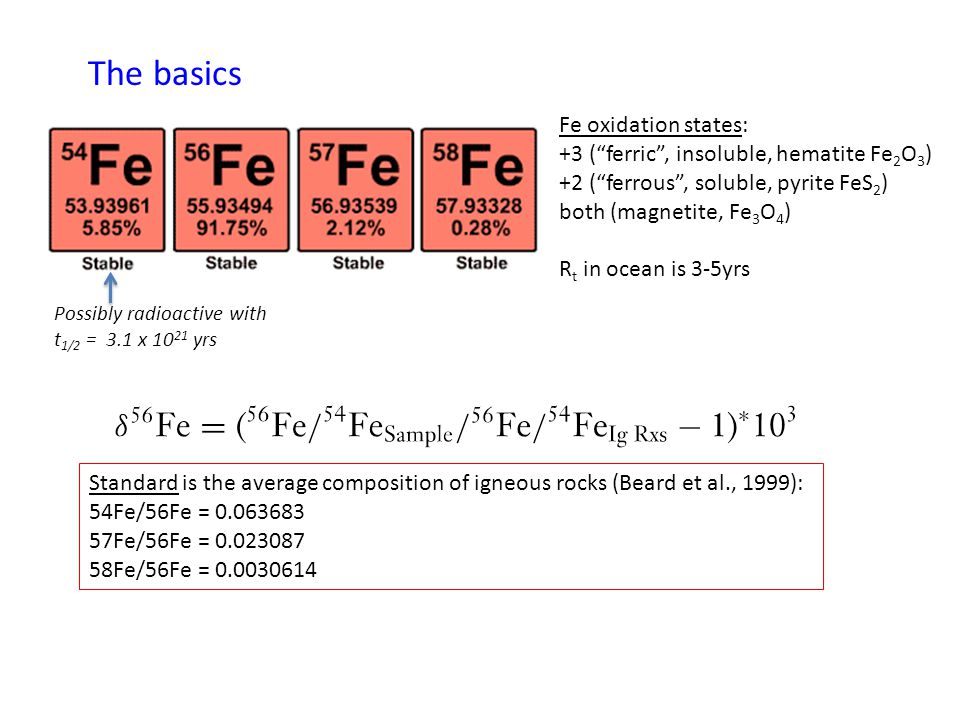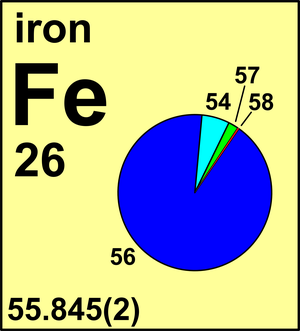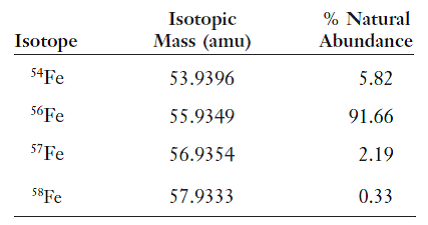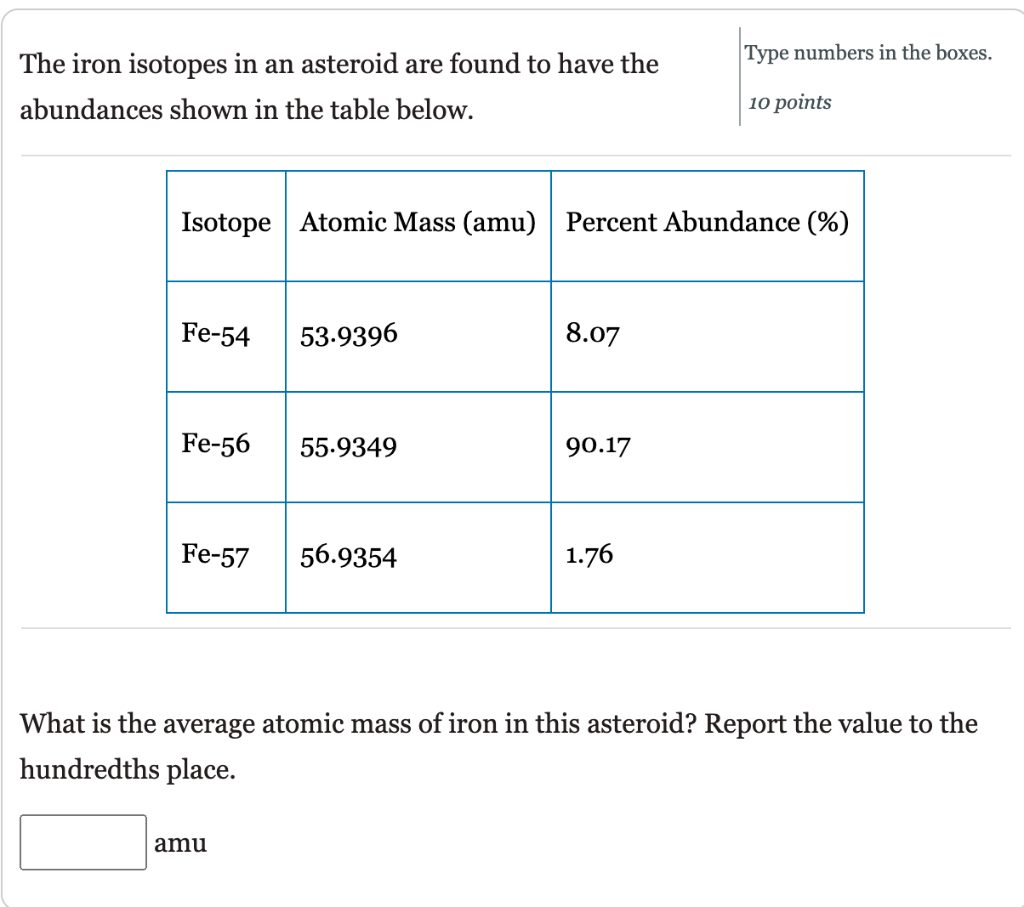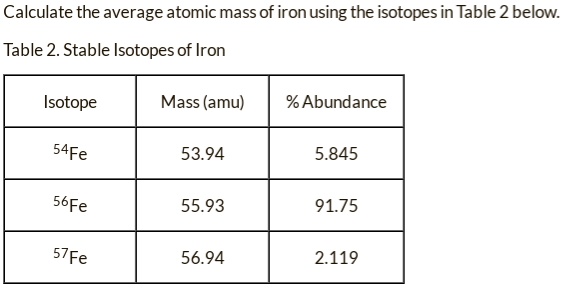
SOLVED: Calculate the average atomic mass of iron using the isotopes in Table 2 below: Table 2. Stable Isotopes of Iron Isotope Mass (amu) % Abundance 54Fe 53.94 5.845 56Fe 55.93 91.75 57Fe 56.94 2.119
![CHEMISTRY; I'm not quite sure what I'm doing. [University Chemistry:Elements] what is the percent abundance of the rarest of these three isotopes of iron? : r/HomeworkHelp CHEMISTRY; I'm not quite sure what I'm doing. [University Chemistry:Elements] what is the percent abundance of the rarest of these three isotopes of iron? : r/HomeworkHelp](https://preview.redd.it/1mzcw4zdk4291.jpg?width=640&crop=smart&auto=webp&s=c33f44ee48508606efd795243fde7444e5a854d6)
CHEMISTRY; I'm not quite sure what I'm doing. [University Chemistry:Elements] what is the percent abundance of the rarest of these three isotopes of iron? : r/HomeworkHelp

Naturally occurring iron consists of four isotopes with the abundances indicated here. From the masses and relative abundances of these isotopes, calculate the atomic weight of naturally occurring iron. | Homework.Study.com

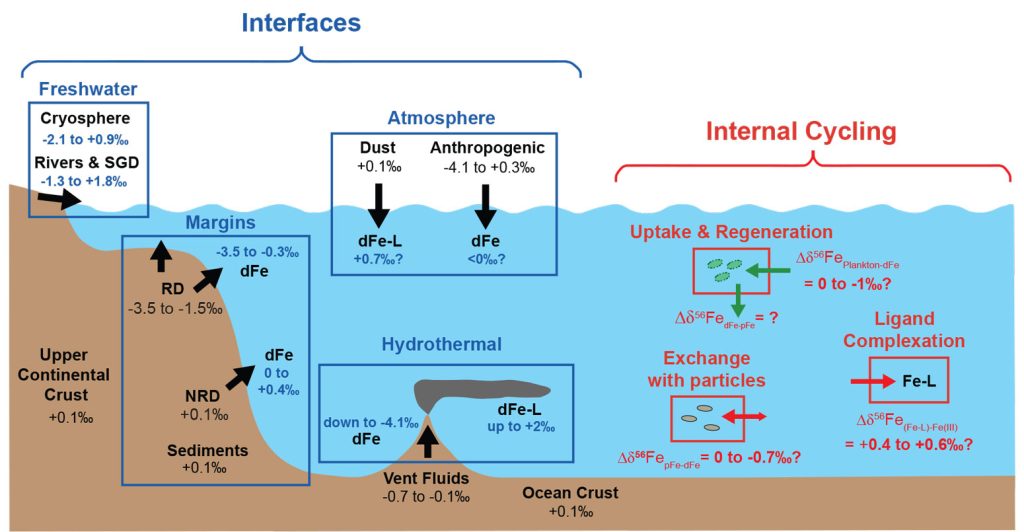
Large Fractionation in Iron Isotopes Implicates Metabolic Pathways for Iron Cycling in Boreal Shield Lakes | Environmental Science & Technology

Formation pathways of Precambrian sedimentary pyrite: Insights from in situ Fe isotopes - ScienceDirect

Iron isotopes trace primordial magma ocean cumulates melting in Earth's upper mantle | Science Advances

Calculating Particles for an ion. Representations from the Periodic Table Fe Iron Oxidation States Name Atomic Mass Atomic Number. - ppt download

Given that the abundances of isotopes ^54Fe, ^56Fe and ^57Fe are 5%, 90% and 5% respectively, the atomic mass of Fe is:

Given that the abundances of isotopes ^54Fe, ^56Fe and ^57Fe are 5%, 90% and 5% respectively, the atomic mass of Fe is: