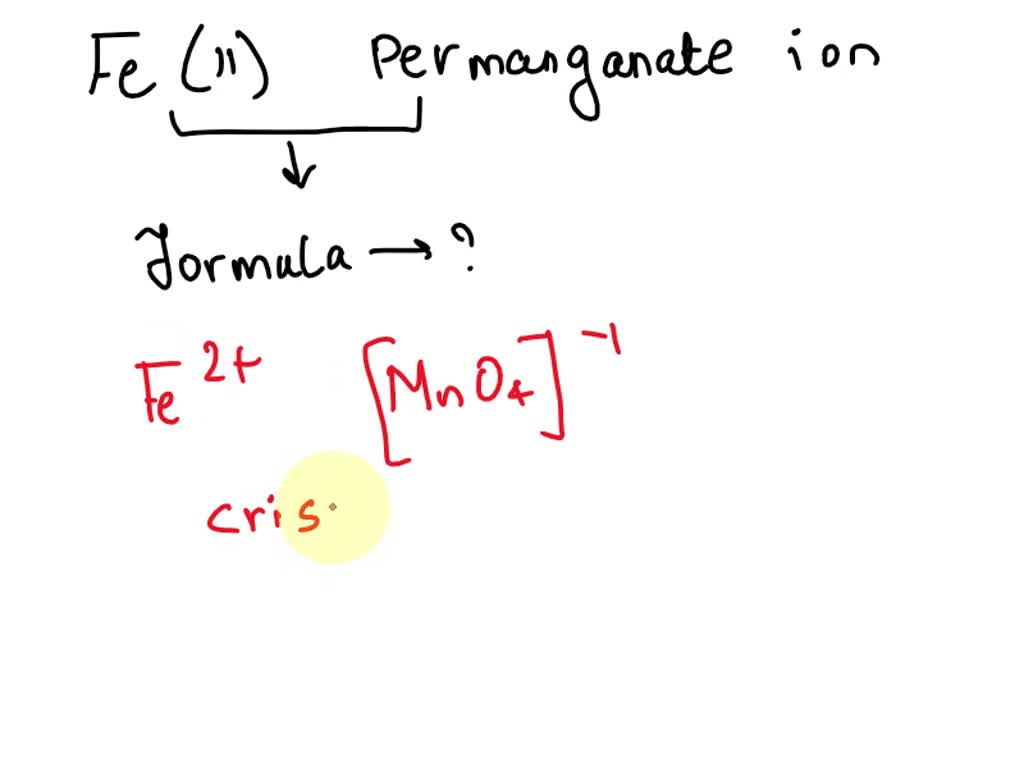
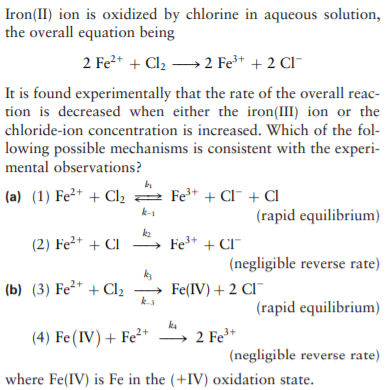
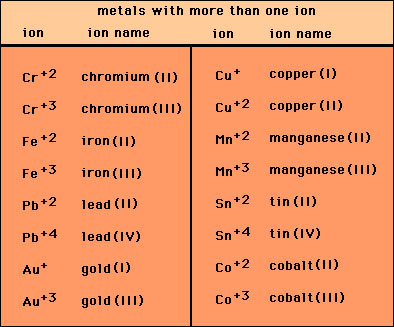
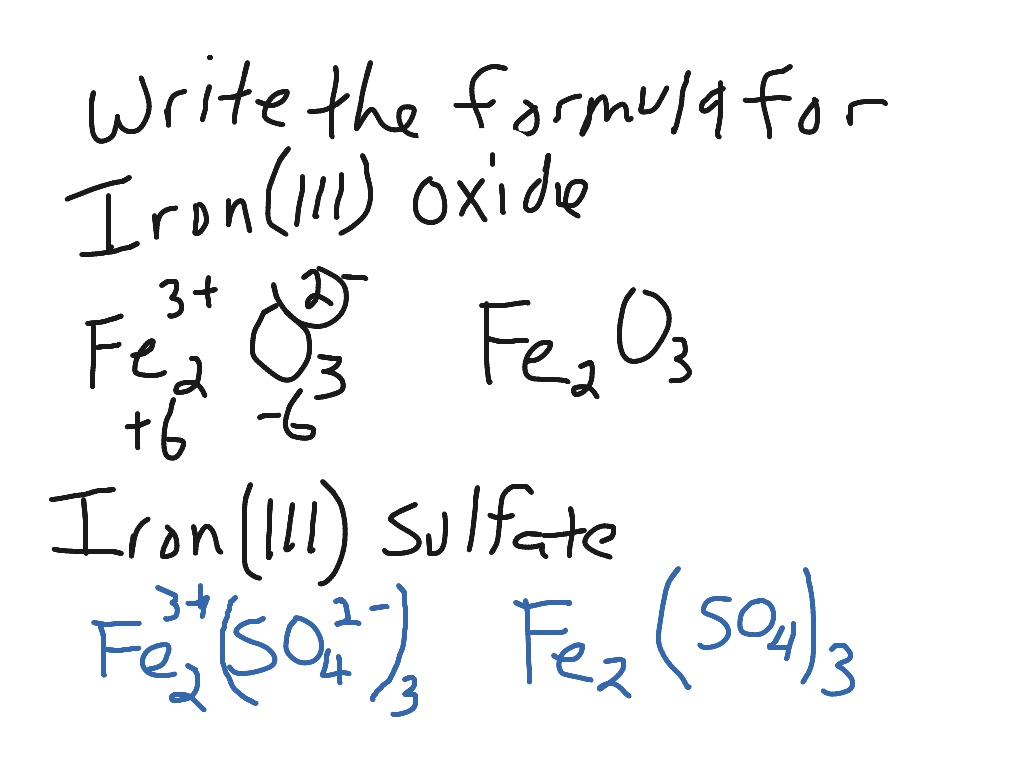Question Video: Deducing the Ionic Formula of an Ionic Compound Where Both Ions Have Greater-Than-One Charge | Nagwa

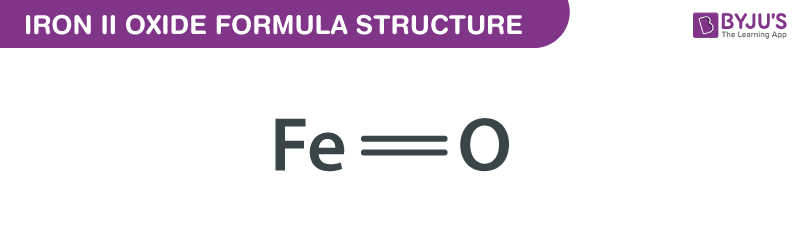
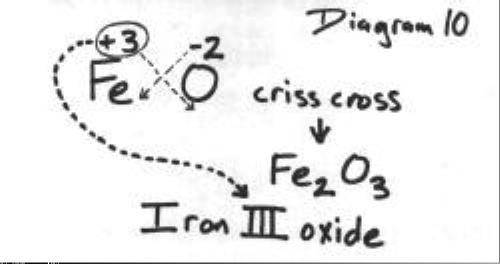
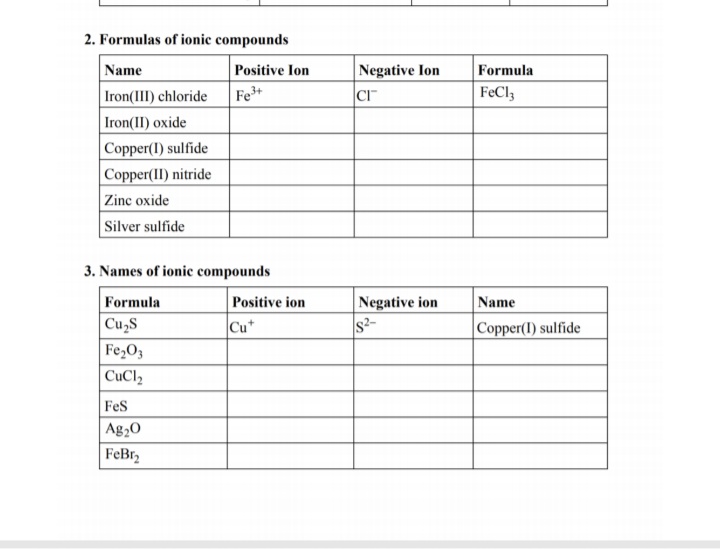
SOLVED: Formulas of Ionic Compounds Name Positive Ion Negative Ion Formula FeCl3 Iron(III) chloride FeO Iron(II) oxide CuS Copper(I) sulfide Cu(NO3)2 Copper(II) nitrate ZnO Zinc oxide Ag2S Silver sulfide 3. Names of