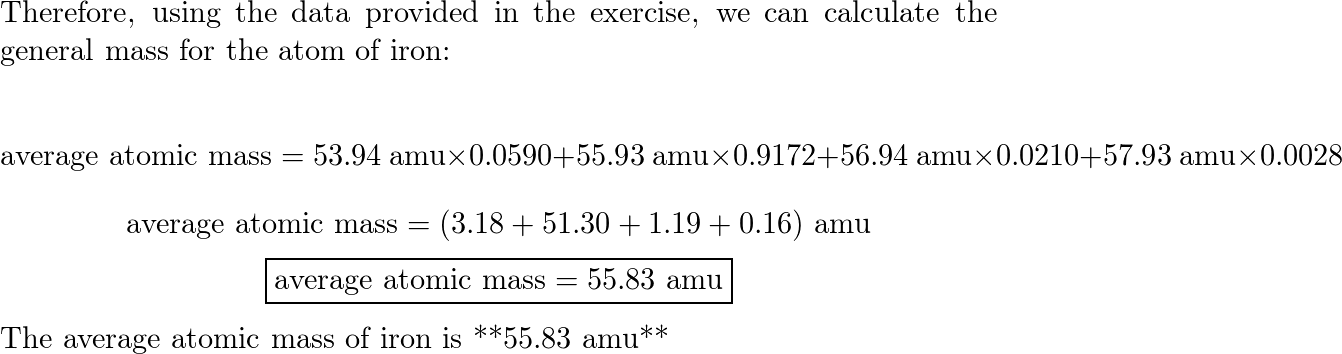1.2 × 10^24 atoms of iron equals moles (Atomic number (Z) and atomic mass of Iron, Fe is 26 and 56 respectively)

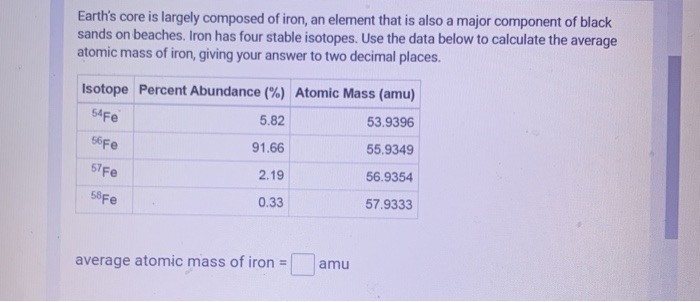
Naturally occurring iron consists of four isotopes with the abundances indicated here. From the masses and relative abundances of these isotopes, calculate the atomic weight of naturally occurring iron. | Homework.Study.com

Calculate the number of iron atoms in a piece of iron weighing `2.8 g` (Atomic mass of iron `= 56 u` - YouTube
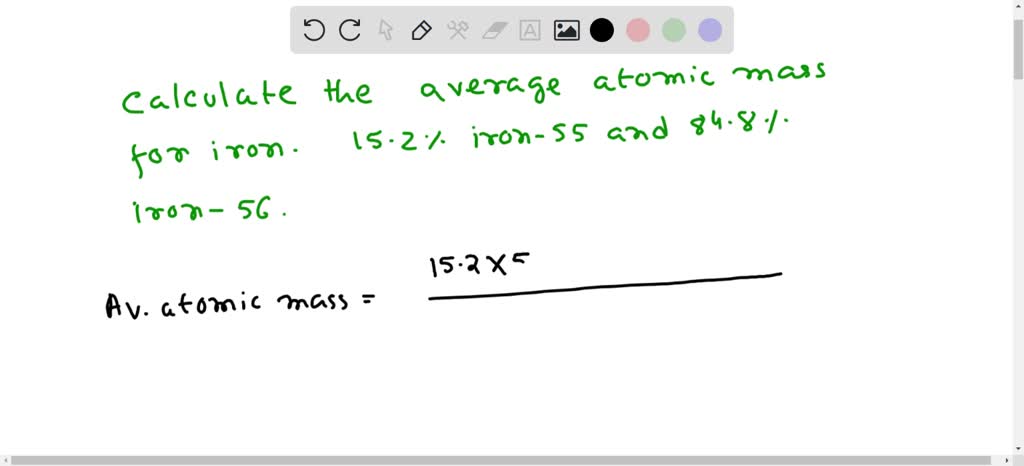
SOLVED: Calculate the average atomic mass for iron. 15.2% iron-55, 84.8% iron-56. AAM = 55(0.152) + 56(0.848)
SOLVED: Calculate the average atomic mass for iron. 15.2% iron-55, 84.8% iron-56. AAM = 55(0.152) + 56(0.848)

Given that the abundances of isotopes ^54Fe, ^56Fe and ^57Fe are 5%, 90% and 5% respectively, the atomic mass of Fe is:
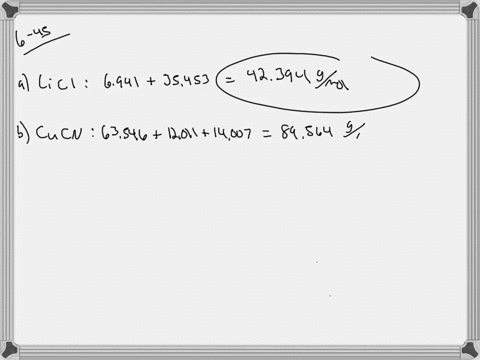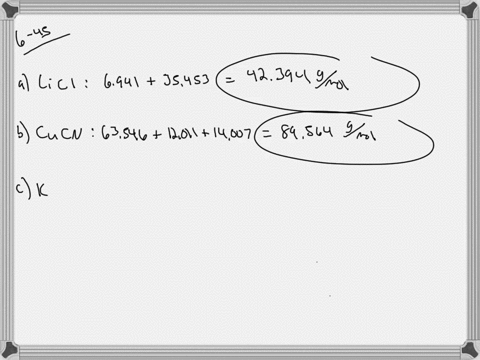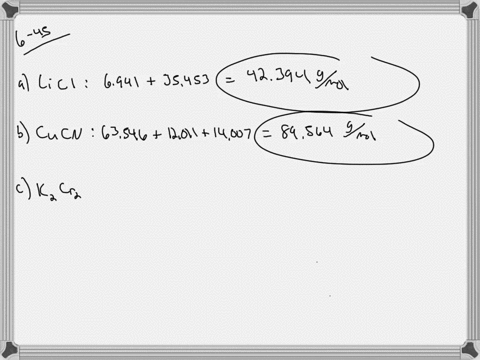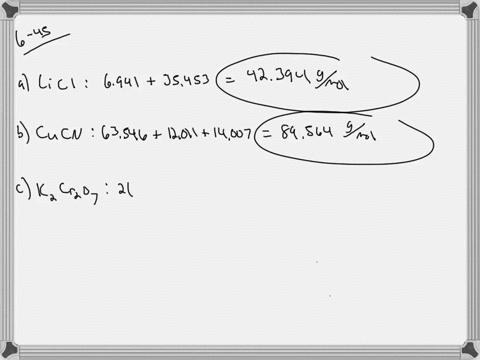
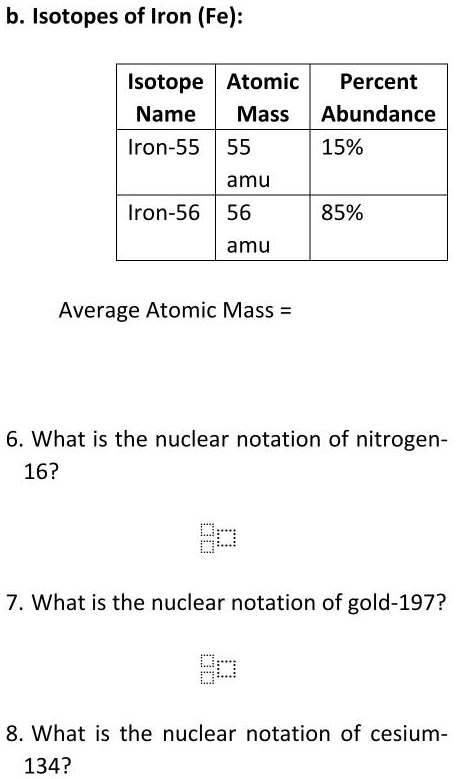
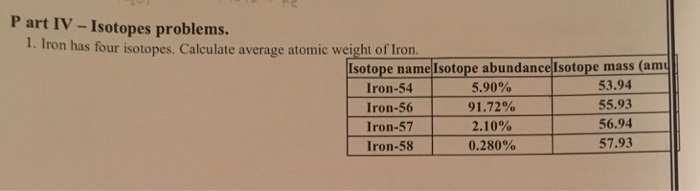
SOLVED:Calculate the average atomic mass of iron. Its composition is 5.90% with a mass of 53.94 amu, 91.72% with a mass of 55.93 amu, 2.10% with a mass of 56.94 amu, and
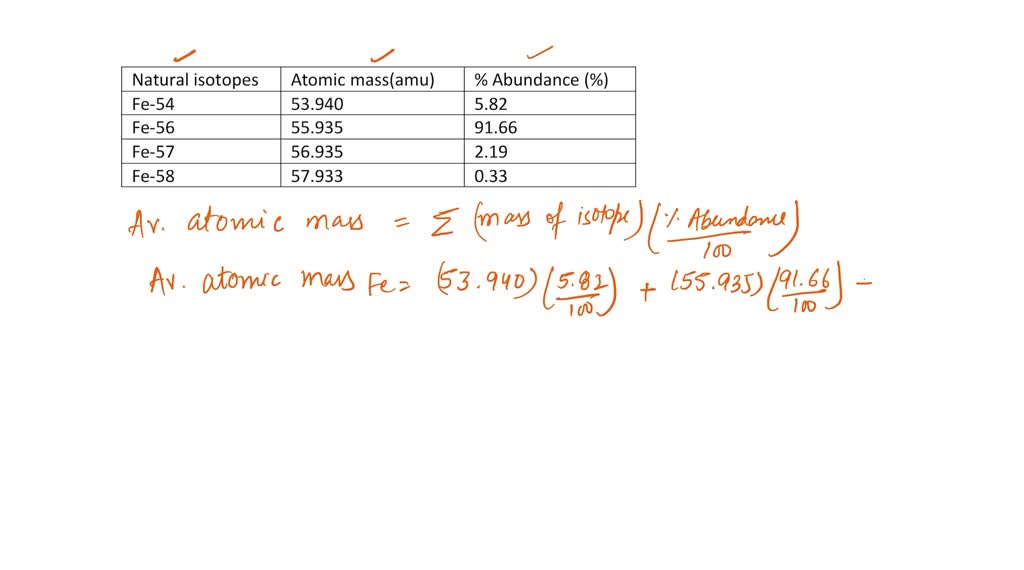
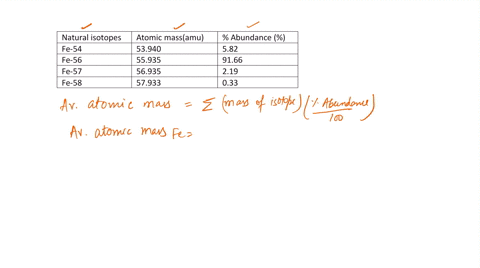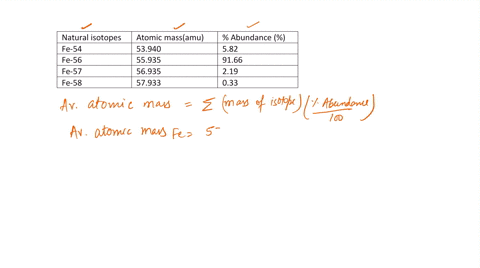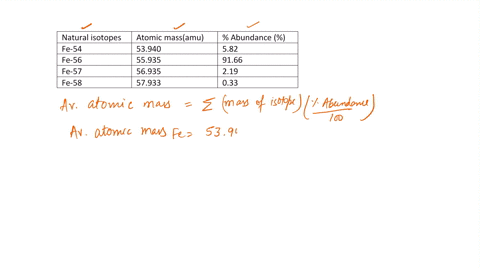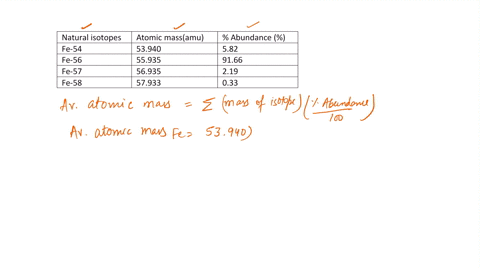
SOLVED: Calculate the atomic mass for iron given the data for its natural isotopes. Fe-54: 53.940 amu 5.82% Fe-56: 55.935 amu 91.66% Fe-57: 56.935 amu 2.19% Fe-58: 57.933 amu 0.33%
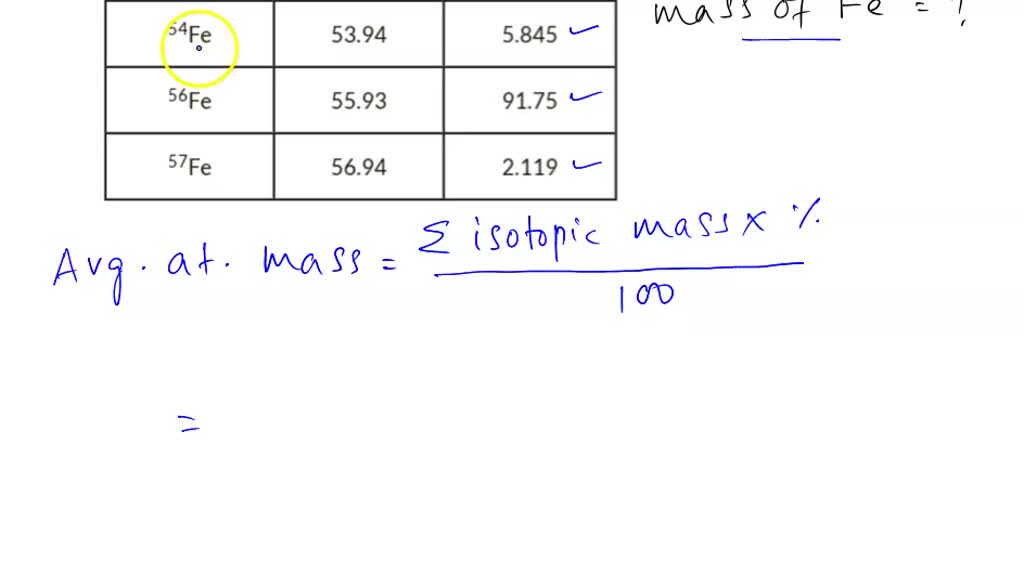
SOLVED: Calculate the average atomic mass of iron using the isotopes in Table 2 below: Table 2. Stable Isotopes of Iron Isotope Mass (amu) % Abundance 54Fe 53.94 5.845 56Fe 55.93 91.75 57Fe 56.94 2.119
Given that natural sample of iron has isotopes Fe,Fe and Fe in the ratio of 5%, 90% and 5% respectively. What will be the average atomic mass of iron ( Fe)? - CBSE
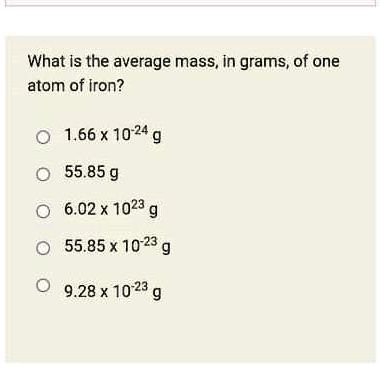
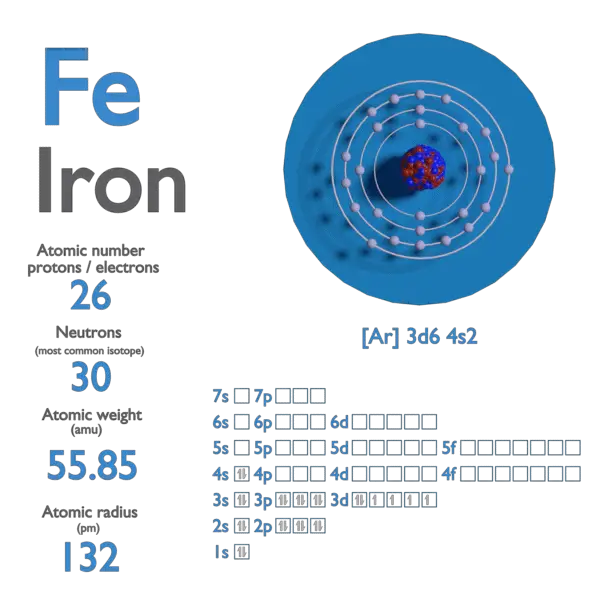
SOLVED: What Is the average mass, In grams; of one atom of Iron? 1.66 x 10-24 g 55.85 g 6.02 x '1023 55.85 x 10-23 g 9.28 x 10.23 g